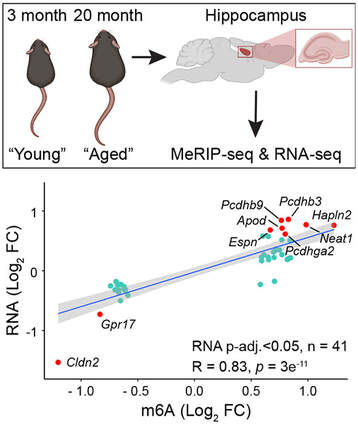
 Our new research describing the m6A-epitranscriptomic profiles from the hippocampi of young (3 month-old) vs aged (20 month-old) C57Bl/6 mice is now out in Aging Cell. We reveal more over 500 transcripts that are differentially methylated. There is also a significant concordance between m6A and transcript levels in both directions. Finally, we found that the myelin regulator gene Gpr17 was downregulated in the aged hippocampus concomitant with reduced m6A levels in its 3'UTR. Overall, the positive correlation between m6A and the transcript expression levels indicates a co-transcriptional regulation of m6A with gene expression changes that occur in the aged mouse hippocampus. Congratulations to River and Jocelyn who lead the project, as well as to our fantastic collaborators Drs. Renhua Song and Justin Wong (Centenary Institute, Sydney). We are very proud to introduce Dr. River Huang, our lab third PhD graduate. River will soon be taking up a new position as a postdoctoral researcher at The Johns Hopkins University of School of Medicine in Baltimore, USA. Congratulations River and we wish you all the very best!
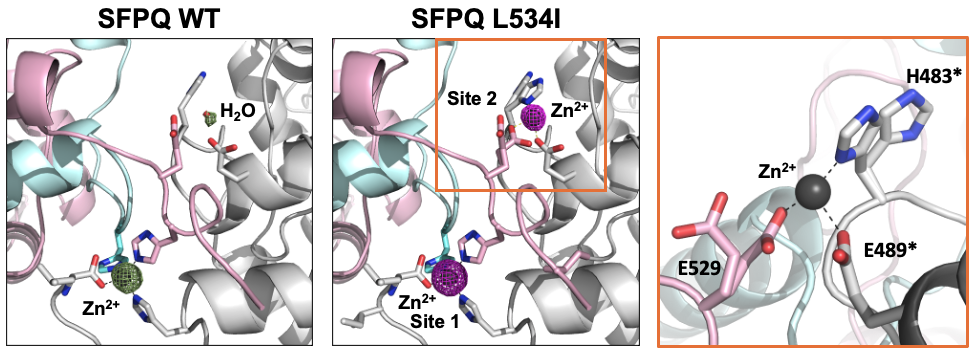
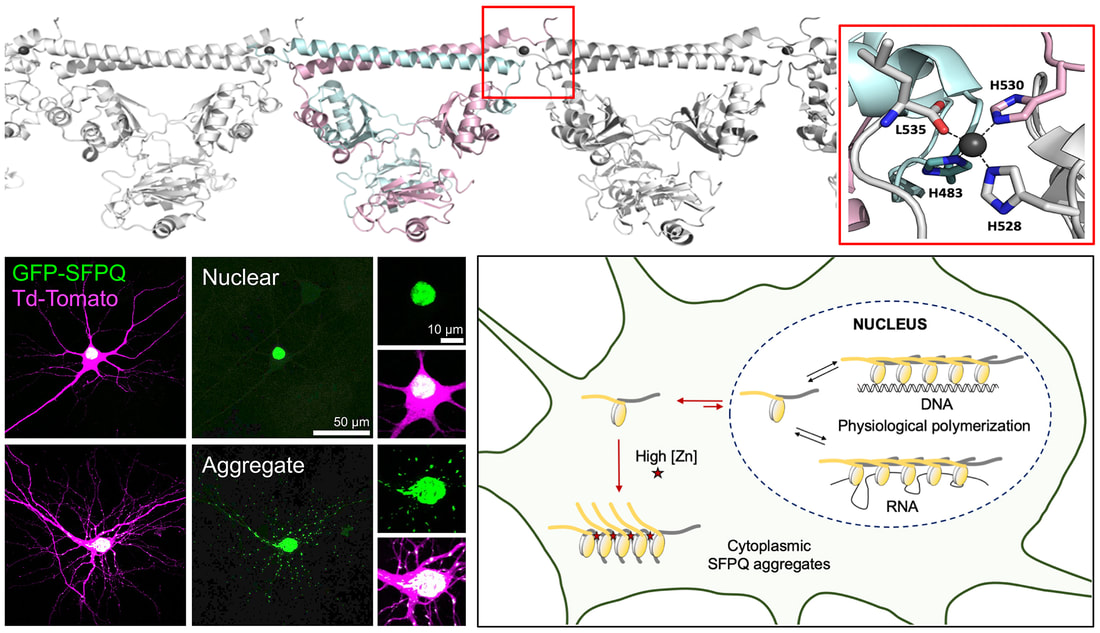
We are delighted to that our new work is now published in Open Biology. Here we reports the structural basis of familial ALS-associated SFPQ variants in promoting the formation of SFPQ cytoplasmic aggregates through enhanced zinc binding. We also found that these aggregates alter the expression of AMPA receptors on the plasma membrane of primary neurons, a phenotype that is commonly found in ALS patients. This is another great collaboration with the lab of Mihwa Lee (La Trobe University) and is an extension of our previous work published in Nucleic Acids Research. Well done Jocelyn, Nishita, Anson and Lara for contributing to this study. Thanks to MND Research Australia (Judy Mitchell MND Research Innovator Grant) for supporting this research.
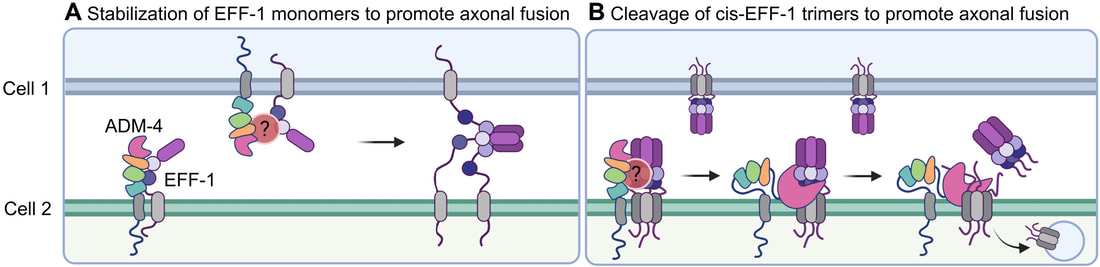
A collaborative study led by Dr Xue Yan Ho from the Hilliard's Lab (QBI, UQ), has identified a molecule essential for regulating the repair of injured nerves, which could help people recover from nerve damage. Xue Yan found that the metalloprotease ADM-4/ADAM17 promotes regenerative axonal fusion by stabilizing the fusogen EFF-1. The paper was published in the journal Science Advances. A great collaboration with Prof. Massimo Hilliard, Dr Sean Coakley and Dr Rumelo Amor. [Press Release]
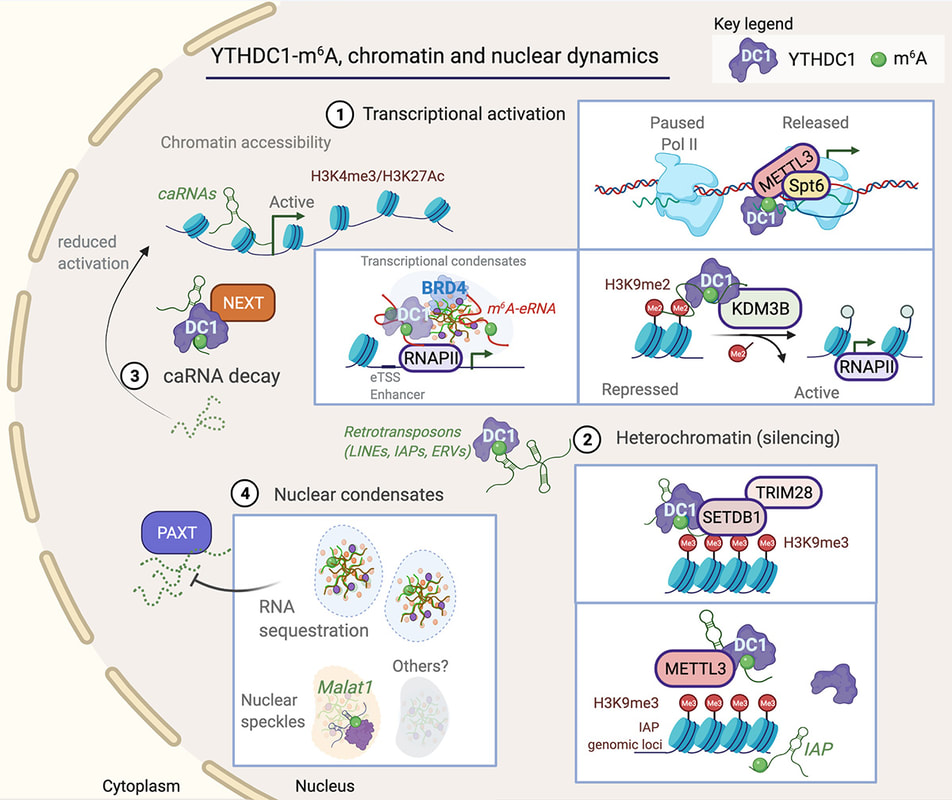
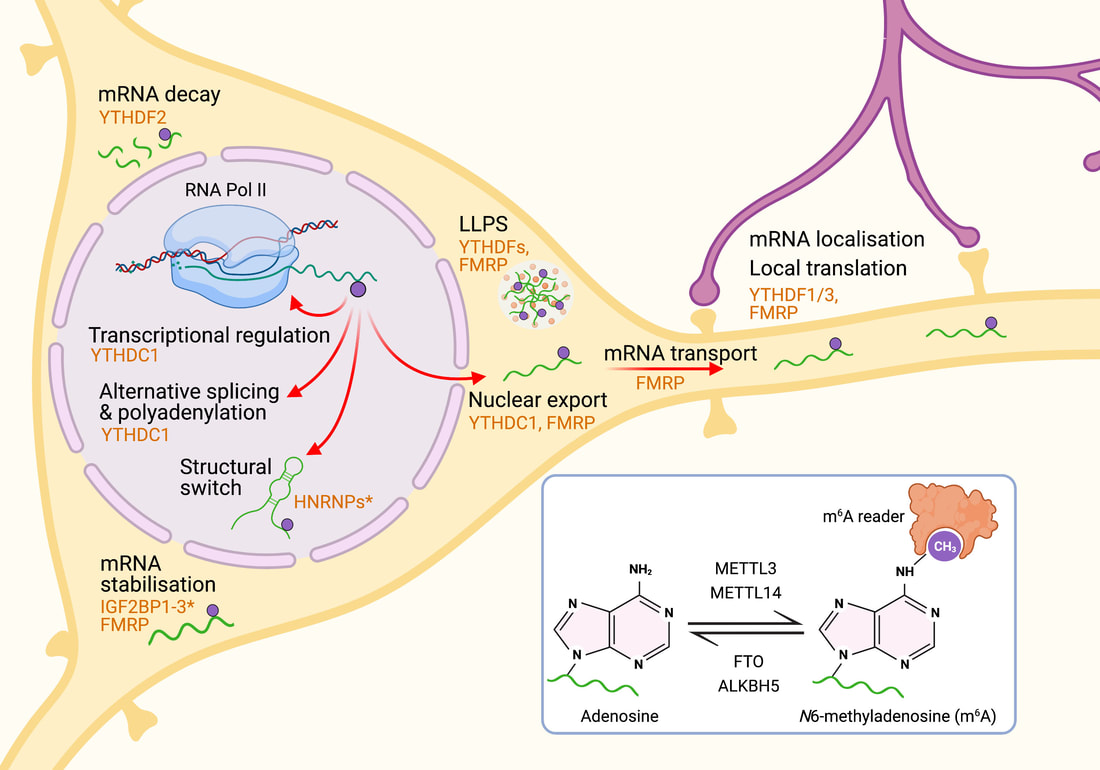
Jocelyn wrote an opinion piece that was published in Trends in Genetics. We propose a unifying mechanism that explains the complexity of YTHDC1 (the nuclear m6A reader) in regulating gene expression. Another great collaboration with Dr Justin Wong (Centenary Institute, Sydney).
Happy to share our new review article by Jocelyn on the m6A regulatory mechanisms of brain plasticity, learning and memory, which has just been accepted for publication in Seminars in Cell and Developmental Biology. A great collaboration with Dr. Justin Wong (Centenary Institute, Sydney).
The lab attended the Clem Jones Centre for Ageing Dementia Research annual retreat at the beautiful Custom House. We were very pleased that our students, Hilary and River, received the best research student publication award (Yong et al., Cell Reports, 2020) and the student's lay abstract presentation people's choice award, respectively. Congratulations!
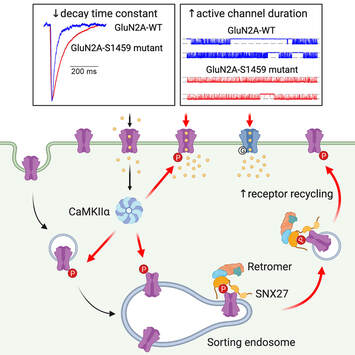
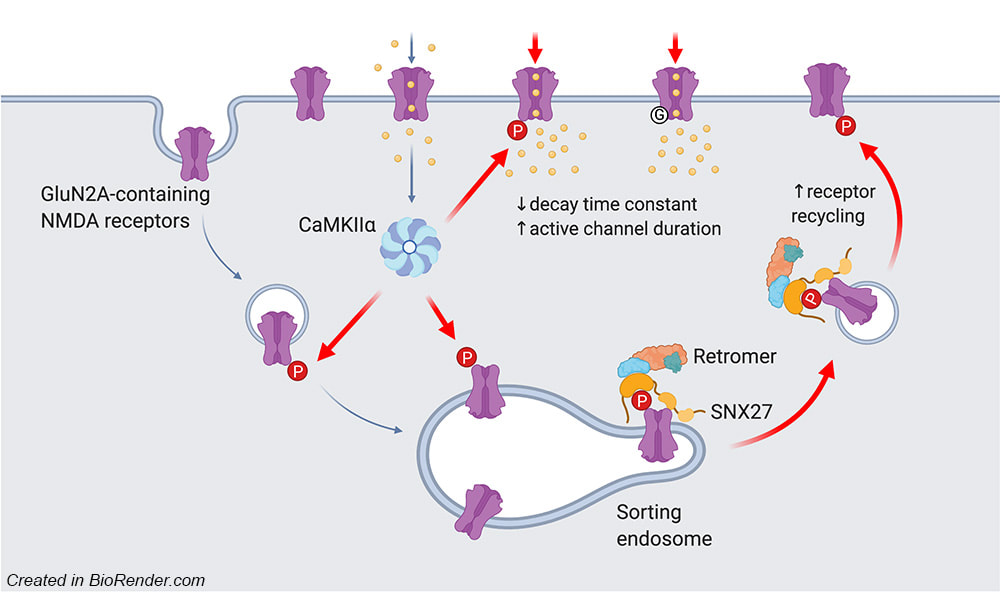
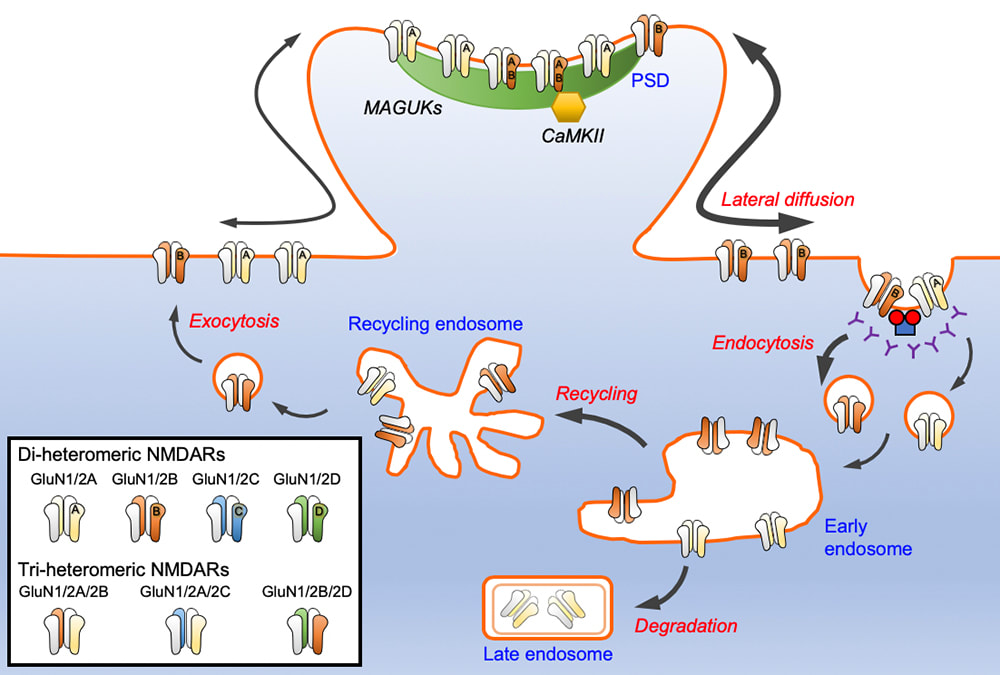
We are very excited to share our lab very first pre-print on bioRxiv. This work, led by our PhD student Hilary Yong, identified functional roles of CaMKIIα-mediated phosphorylation on the C-terminal tail of the GluN2A subunit (Ser-1459) in regulating the gating and activity-dependent trafficking of NMDA receptors. It is an extension of our recent collaborative work that was published in Cell Reports (Vieira et al., 2020). We thank all authors and our wonderful collaborators, Prof. Brett Collins (IMB, UQ), Dr. Angelo Keramidas (IMB, UQ), Prof. Joe Lynch (QBI, UQ) and Prof. Katherine Roche (NINDS, NIH, USA).
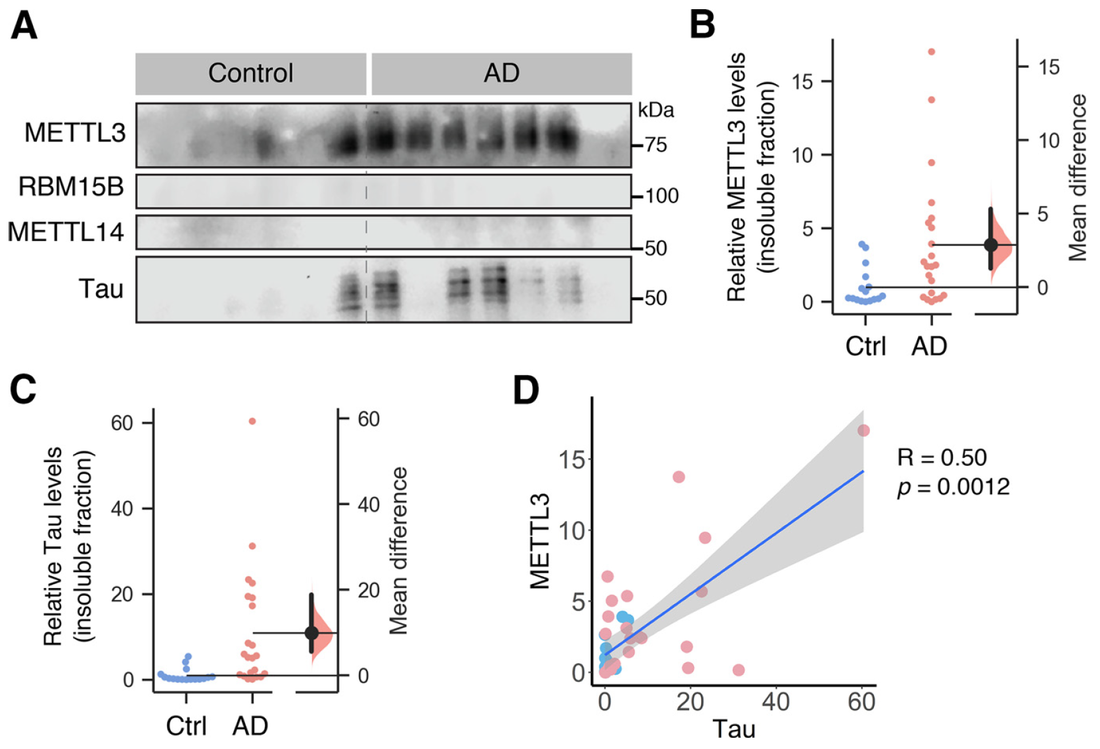
 Even though 2020 has been a tough year, we have performed well with 7 published papers, two of which were led by PhD students, River and Hilary. To cap off the year, the lab was awarded the Judy Mitchell MND Research Innovator Grant by the MND Research Australia. This grant was jointly awarded to Victor, Jocelyn and our collaborator, Dr. Mihwa Lee (La Trobe University) to investigate molecular mechanisms underlying the cytoplasmic aggregation of the RNA binding protein, SFPQ, in ALS. Finally, we are very proud of Hilary, whose work on PICK1 in synaptic vesicle recycling (Yong et al., Cell Reports, 2020) was selected as the Runner Up of QBI Best Student Publication Prize 2020. We wish everyone a happy festive season and all the very best in 2021. 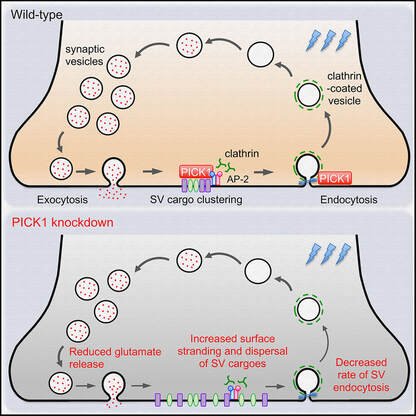 Our latest work has just been published in Cell Reports showing a new role for PICK1 (the only protein with a BAR and a PDZ domain) in regulating the activity-dependent clustering and retrieval of presynaptic vesicle cargo in mammalian central neurons. It is required for efficient synaptic vesicle endocytosis and sustained glutamate release. Outstanding work by Hilary and a wonderful collaboration with Prof. Mike Cousin (The University of Edinburgh, UK). 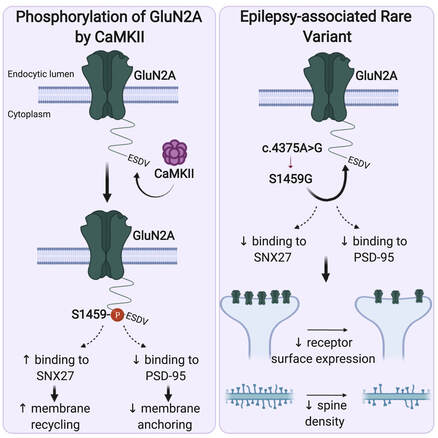 A new collaborative study led by the Roche's Lab at the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), is now published in Cell Reports. This study identified CaMKIIα phosphorylation of the GluN2A subunit on Ser-1459 as a mechanism regulating NMDA receptor trafficking. An epilepsy-associated rare variant at this same residue, GluN2A-S1459G, results in altered protein interactions, decreased NMDA receptor surface expression, and reduced synaptic function, providing potential insight into an epilepsy phenotype. Congratulations to the lead author, Dr. Marta Vieira, and Katherine on this beautiful work. We are excited to share our new work, "Altered expression of the m6A methyltransferase METTL3 in Alzheimer's disease", which has just been published in eNeuro. Here we report a decrease in the expression of METTL3 mRNA and soluble protein in the postmortem hippocampal tissues of Alzheimer's disease (AD) patients. We also identified a striking alteration in the METLL3 protein expression, including enhanced insolubility and immunoreactivity in the AD hippocampus. Our results suggest that perturbation of m6A signalling may present a novel cellular mechanisms underpinning dysregulation of gene expression associated with AD pathophysiology. Congratulations to River and Jocelyn who led this study and to our QBI collaborators, Drs. Judith Camats-Perna and Rodrigo Medeiros.
We wish to congratulate the following lab members for their achievements:
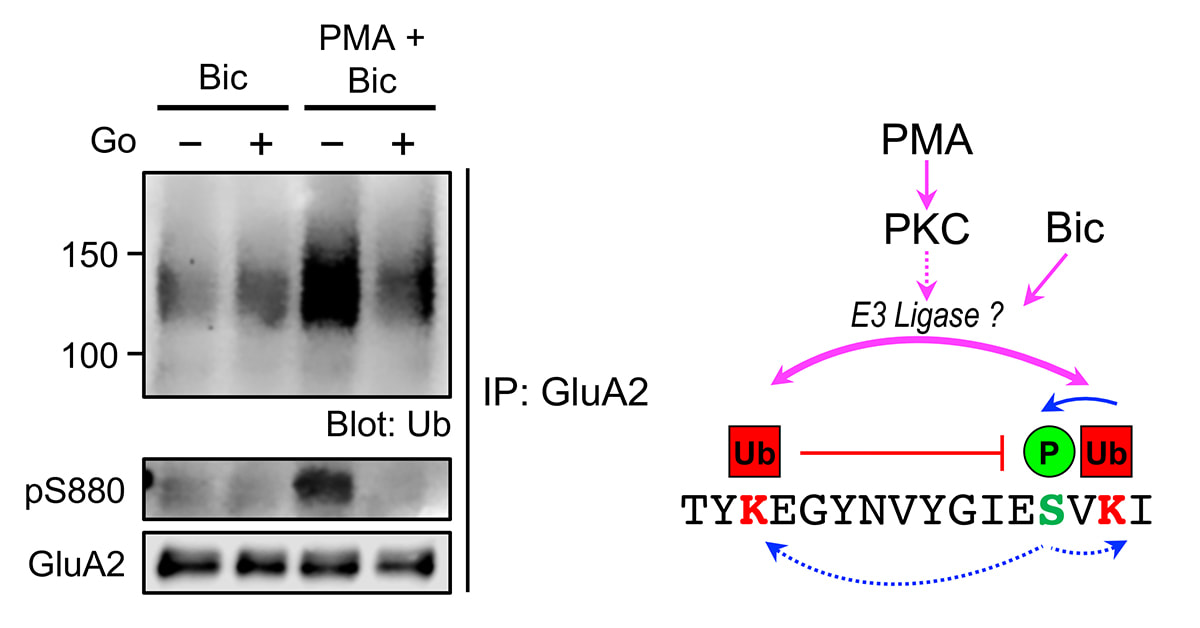
We are delighted that our study, primarily done by Jocelyn and Jun Wei, has just been published in Cellular and Molecular Neurobiology. Here, we demonstrated the cross-modulation between the ubiquitination and phosphorylation of the GluA2 subunit of AMPA receptors by protein kinase C (PKC). Interestingly, this effect is specific for GluA2 as phorbol ester does not potentiate bicuculline-induced ubiquitination of the GluA1 subunit. We envisage that the binding of glutamate on AMPARs in neurons with high level of PKC activity (such as following the activation of mGluRs) will result in subunit-specific regulation of AMPAR ubiquitination and intracellular sorting, which ultimately govern the subunit composition and number of AMPARs, including the Ca2+-permeable AMPARs at synapses.
SFPQ is an abundant and ubiquitous nuclear RNA-binding protein (RBP) that has been implicated in gene regulation and subnuclear body formation. In a study led by Dr. Mihwa Lee (La Trobe University, Melbourne), we report the crystal structure of SFPQ in complex with Zn(II), which reveals an infinite polymer of SFPQ mediated by Zn(II) binding to the protein. The application of Zn(II) to primary cortical neurons induced the cytoplasmic accumulation and aggregation of SFPQ. Mutagenesis of the three Zn(II)-coordinating histidine residues resulted in a significant reduction in the zinc-binding affinity of SFPQ in solution and the Zn(II)-induced cytoplasmic aggregation of SFPQ in cultured neurons. This study, which was published in Nucleic Acids Research, offers a new framework for how metal-induced polymerization of RBPs can induce cytoplasmic aggregation, which are commonly associated with neurodegenerative diseases.
The N-methyl-D-aspartate glutamate receptors (NMDARs) mediate calcium-dependent signaling that underpins multiple forms of synaptic plasticity. Different GluN2 (GluN2A-D) subunit confers NMDARs with distinct ion channel properties and intracellular trafficking pathways. In a review article which has just been published in Journal of Neurochemistry, we discuss the current knowledge of the molecular mechanisms that regulate the trafficking of GluN2-containing NMDARs, focusing on the roles of several key synaptic proteins that interact via their carboxyl termini. This review article is a joint effort with our collaborator Prof. Katherine Roche (NINDS, NIH). Congratulations to Marta (Roche Lab) and Hilary (Anggono Lab) on an excellent work.
We are excited to welcome Dr. Anson Tan (postdoctoral fellow) and Mr. Sooraj Das (PhD student), who have just joined the Anggono Lab to investigate the mechanisms of glutamate receptor trafficking in neurons. Anson received his PhD from the University of Melbourne studying the mechanisms of APP trafficking under the supervision of Prof. Paul Gleeson, while Sooraj received his BS-MS double degree from the Indian Institute of Science Education and Research (IISER) Pune, India. We are also very fortunate to retain Ms Hilary Yong (former Research Assistant), who will pursue her PhD degree in the lab. Both Sooraj and Hilary received highly competitive Research Training Program Scholarships from the Australian Government.
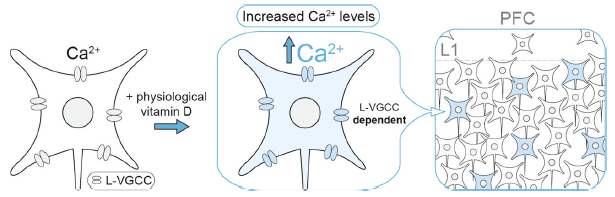
Genetic variants in genes encoding L-type voltage-gated calcium channel (L-VGCC) subtypes are associated with increased risk for schizophrenia. Likewise, epidemiological study has implicated developmental vitamin D deficiency as a risk factor for schizophrenia. In the latest study published in Translational Psychiatry, we (led by Prof. John McGrath of QBI, UQ and Aarhus University, Denmark) showed that the active vitamin D metabolite exert a rapid, non-genomic modulation of L-VGCCs in a subset of neurons in developing medial prefrontal cortex in mice. Optimal modulation of L-VGCCs by 1,25(OH)2-vitamin D may therefore contribute to the healthy development of Vitamin D-responsive neurons within the maturing cortical circuits.
A collaborative study led by a PhD student, Eunice Wong from the Degnan's Labs (School of Biological Sciences, UQ), has revealed ancient submodules of co-expressed "synaptic gene" orthologues during development and in cell type-specific manners. Although synapses do not exist in the sponge, these submodules may contribute to sensory roles in specific cell types in the sponge. The paper was published in the journal Scientific Reports.
We are delighted to welcome Mr. Liming Yang, a third year Bachelor of Medical Science student from the University of Exeter, UK. Liming will undertake a one-year research in the Anggono Lab as part of the Professional Training Year programme.
Syntaxin1A neomorphic mutations promote rapid recovery from isoflurane anesthesia in fruit flies22/7/2019
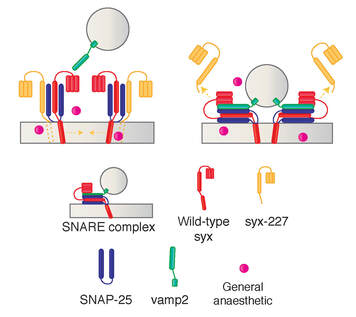 A collaborative study from the van Swinderen laboratory (QBI, UQ) published in Anesthesiology, has shown that transiently expressing a truncated syntaxin1A (Δ227) in adult Drosophila flies facilitates recovery from isoflurane anesthesia. Interestingly, our biochemical study revealed that the truncated syntaxin1A is absence from the presynaptic SNARE complex, suggesting that the resistance-promoting effect occurs prior to SNARE formation. We are delighted to host Dr. Rosie Bamford from the Oguro-Ando's Lab, our collaborator at the Exeter University, UK for one month. Rosie was awarded a prestigious Churchill Fellowship, which supported her trip to Brisbane. Congratulations and welcome to the lab.
|
Synaptic Neurobiology LabArchives
January 2023
Categories
All
|



 RSS Feed
RSS Feed