|
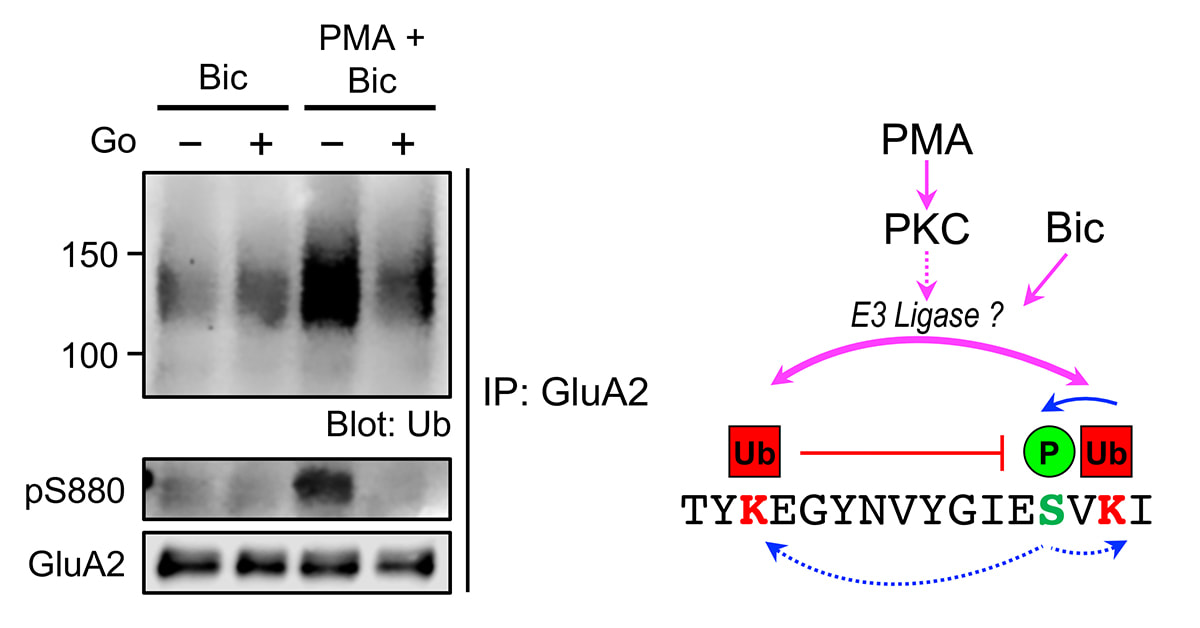
We are delighted that our study, primarily done by Jocelyn and Jun Wei, has just been published in Cellular and Molecular Neurobiology. Here, we demonstrated the cross-modulation between the ubiquitination and phosphorylation of the GluA2 subunit of AMPA receptors by protein kinase C (PKC). Interestingly, this effect is specific for GluA2 as phorbol ester does not potentiate bicuculline-induced ubiquitination of the GluA1 subunit. We envisage that the binding of glutamate on AMPARs in neurons with high level of PKC activity (such as following the activation of mGluRs) will result in subunit-specific regulation of AMPAR ubiquitination and intracellular sorting, which ultimately govern the subunit composition and number of AMPARs, including the Ca2+-permeable AMPARs at synapses.
Comments are closed.
|
Synaptic Neurobiology LabArchives
January 2023
Categories
All
|
 RSS Feed
RSS Feed