ABOUT USSynaptic Neurobiology LabThe brain capacity to learn and remember grants the life experience and survival of living organisms. While there has been great advance in the field of neuroscience, there are still many unresolved questions at the molecular and system levels. Our laboratory aims to unravel novel molecular pathways that underlie neuronal plasticity during the process of learning and memory. Consequently, some of these pathways can help our understanding of the basis of cognitive deficit and neurological disorders.
|
PROJECTSFrom synapses to nuclear signallingThe ability of neurons to modulate the strength of their connectivity, termed synaptic plasticity, remains the most attractive molecular correlate of learning and memory. Our laboratory integrates biochemical, molecular, cellular, and behavioural approaches, to gain deeper mechanistic insights of key cellular events in the pre- (presynaptic vesicle recycling) and post-synapses (glutamate receptor trafficking), as well as in the nucleus (RNA-mediated epigenetic regulation) that underpin synaptic plasticity, learning and memory. Ultimately, we aim to understand how dysregulation of these signalling pathways contributes to neurological disorders and neurodegenerative diseases.
|
NEWS
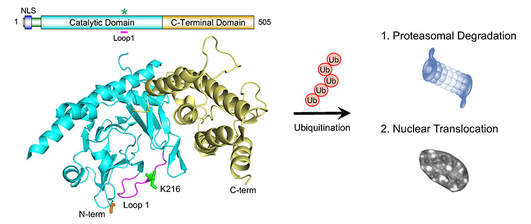
Regulation of FTO function by protein ubiquitination
|
The fat mass and obesity-associated (FTO) protein is the major RNA demethylating enzyme, which has been implicated in the regulation of food intake, energy metabolism and body weight. Humans carrying homozygous loss-of-function FTO alleles displays postnatal growth retardation, polymalformative syndrome and early mortality. Our earlier study has shown a role for FTO in memory consolidation in mice (Widagdo et al., Journal of Neuroscience, 2016). In the latest study published in the Journal of Molecular Biology (Zhu et al., 2018), we report that FTO undergoes post-translational ubiquitination at the evolutionary conserved Lys-216, which in turn regulates the proteostasis and localisation of FTO in cells.
|
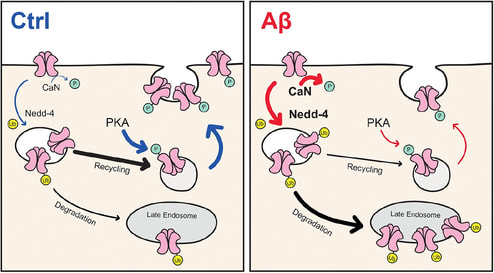
Mechanism of AMPA receptor downregulation by β-amyloid
|
A follow up on our earlier work on AMPA receptor ubiquitination in neurons (Widagdo et al., Cell Reports, 2015), our new paper by Guntupalli et al. reports the involvement of the same ubiquitination pathway in mediating amyloid-beta induced removal of surface AMPA receptors.
This paper is also featured in the Journal of Biological Chemistry virtual issue on Ion Channels. |