|
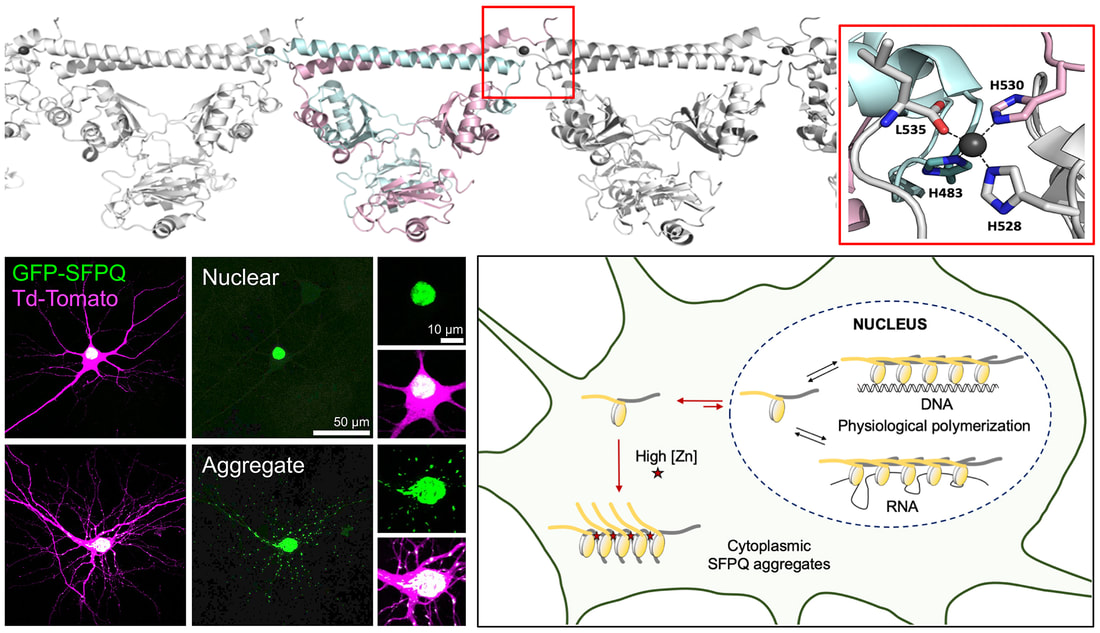
SFPQ is an abundant and ubiquitous nuclear RNA-binding protein (RBP) that has been implicated in gene regulation and subnuclear body formation. In a study led by Dr. Mihwa Lee (La Trobe University, Melbourne), we report the crystal structure of SFPQ in complex with Zn(II), which reveals an infinite polymer of SFPQ mediated by Zn(II) binding to the protein. The application of Zn(II) to primary cortical neurons induced the cytoplasmic accumulation and aggregation of SFPQ. Mutagenesis of the three Zn(II)-coordinating histidine residues resulted in a significant reduction in the zinc-binding affinity of SFPQ in solution and the Zn(II)-induced cytoplasmic aggregation of SFPQ in cultured neurons. This study, which was published in Nucleic Acids Research, offers a new framework for how metal-induced polymerization of RBPs can induce cytoplasmic aggregation, which are commonly associated with neurodegenerative diseases.
Comments are closed.
|
Synaptic Neurobiology LabArchives
January 2023
Categories
All
|
 RSS Feed
RSS Feed